Kiora Pharmaceuticals (NASDAQ: KPRX) KIO-301 is lighting up the eyes of some patients with retinitis pigmentosa (RP) in a Phase 1 clinical study, so much so that “I was blind, and now I can see” is not hyperbole. Kiora is expected to provide an update on the ongoing study in the fourth quarter of 2023, but preliminary data to date are highly encouraging. Kiora is focusing its near-term efforts with KIO-301 on RP, but tremendous potential exists to expand development into other rare, inherited retinal diseases, as well as the potential to monetize additional assets in the pipeline.
RP is a group of inherited eye diseases that cause progressive vision loss. It is characterized by the gradual death of light-sensitive photoreceptor cells in the retina, known as rods and cones, that are responsible for converting light into neural signals sent to the brain. The cell death is the result of an inherited genetic mutation. More than 150 distinct mutations have been linked to the disease to date. RP affects approximately 1 in 4,000 people around the world.
The symptoms of RP usually start in childhood or adolescence. The first symptom is often night blindness, which is the inability to see in dim light. As the disease progresses, people with RP lose their peripheral vision, which is the ability to see things to the sides of their field of vision. This results in tunnel vision. In the later stages, people with RP may only lose their ability to see things directly in front of them, resulting in complete vision loss.

RP is characterized by the progressive loss of photoreceptors (rods and cones). Other retinal neurons, including the retinal ganglion cells (RGCs), are spared. RGCs receive input from photoreceptor cells (via Bipolar Cells) and then transmit this information to the brain via the optic nerve. RGCs can be classified as either ON or OFF cells. ON cells respond to increases in light, while OFF cells respond to decreases in light. Ion channels, including K+ channels (voltage-gated potassium channels) and HCN channels (hyperpolarization-activated cyclic nucleotide-gated channels) in RGCs are responsible for regulating the signaling activity to the brain. These channels help set the resting membrane potential and control the responsiveness of the cells to synaptic input.
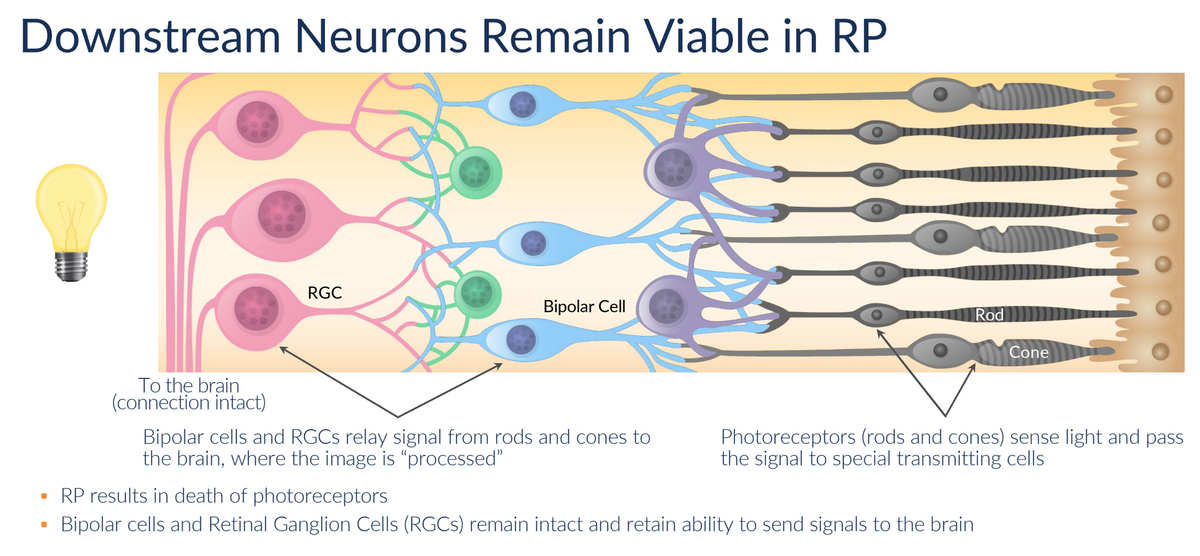

The death of the photoreceptor cells in RP patients results in RGCs being stuck in the OFF state. Kiora has discovered a way to shift RGCs to the ON state in the presence of light. This is where KIO-301 comes into play. KIO-301 preferentially enters RGCs in the retina via the P2X7 transmembrane receptor, which, quite fortuitously, is highly upregulated in these cells. Once inside the cell, KIO-301 lodges inside the Kv/HCN ion channel in a non-covalent (reversible) manner. In the absence of light, KIO-301 is in the trans-configuration, allowing the ion channel's normal flow. This results in the OFF state for RGCs. However, in the presence of light, KIO-301 undergoes a reversible isomeric shift to the cis-configuration, blocking ion efflux through Kv/HCN channels. This triggers RGCs to the ON state, sending a signal to the brain that there is light. This process reverses itself once the source of light is lost.
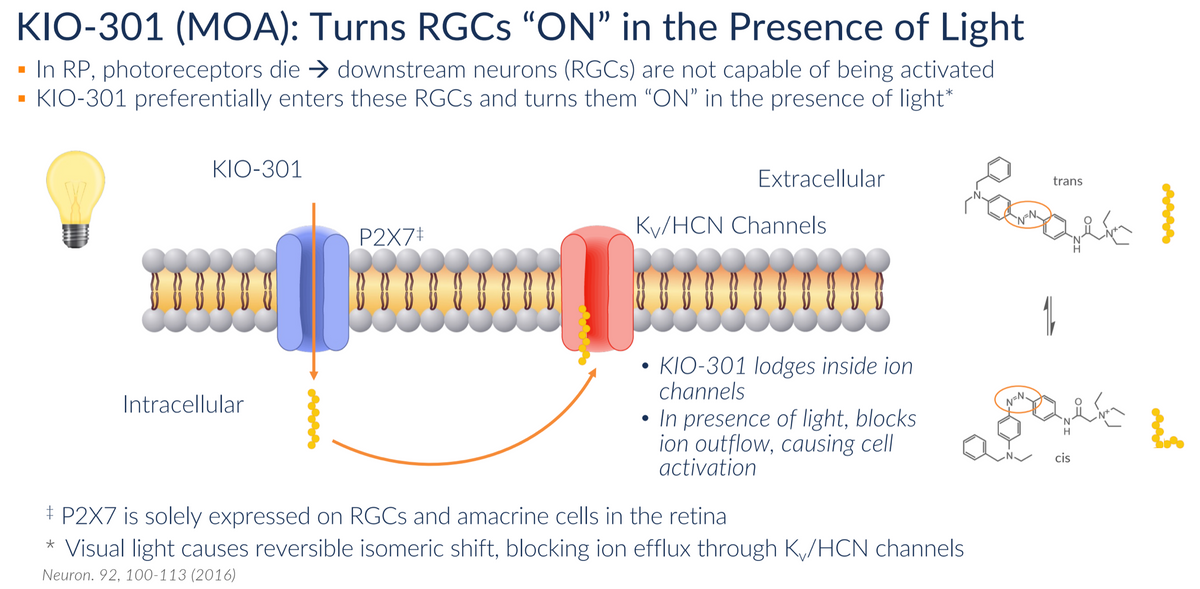
The technology discovery behind the concept of photoswitch molecules came out of the University of California, Berkeley and the University of Washington. What distinguishes KIO-301 from many of the other therapies in development is that it is not a gene therapy. This confers several advantages.
- Mutation agnostic: KIO-301 has the potential to be used in any one of the more than 150 gene mutations associated with Retinitis Pigmentosa. It could also be used in combination with any future gene therapy that may come along.
- Delivery: KIO-301 is administered via intravitreal injection (IVT), which allows for a more consistent and tolerable administration
- Cost: KIO-301 is a small molecule, which can be manufactured and provided to patients at a fraction of the cost of a gene therapy such as Luxturna®, which costs roughly $450,000 per eye.

Kiora is currently conducting a Phase 1b clinical study with KIO-301 in Australia. Australia has proven to be an outstanding place to conduct early-stage clinical research. The country has among the highest-quality healthcare facilities in the world, most notably in sensory organs like the eyes and ears. The country is safe, English-speaking, and highly respected, and companies like Kiora can conduct clinical studies at a reduced cost to the U.S. and confident that the U.S. and European medical community and regulators will accept the results. It’s a great place to conduct credible proof-of-concept clinical work.
Kiora is hoping to complete this Phase 1b study in November of 2023 and present the data to the FDA as part of a collaborative discussion to define the U.S. development path. With solid data in hand from Australia, the potential exists to expedite development in the US. Ocular Therapeutics took a similar route, conducting several of its early-stage clinical studies with its ocular hydrogel implant in Australia before filing U.S. IND applications.
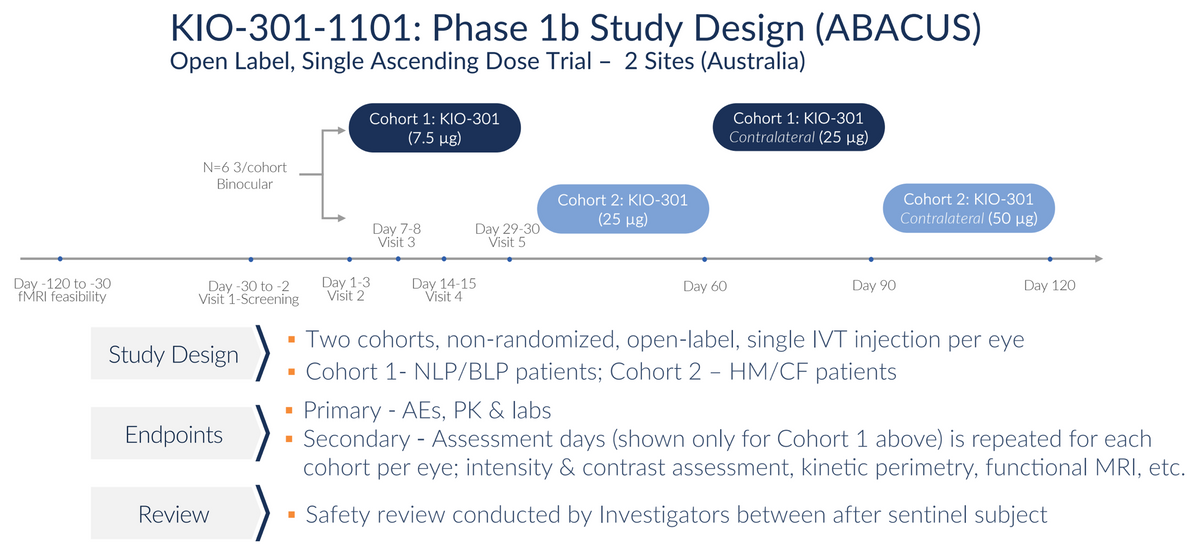
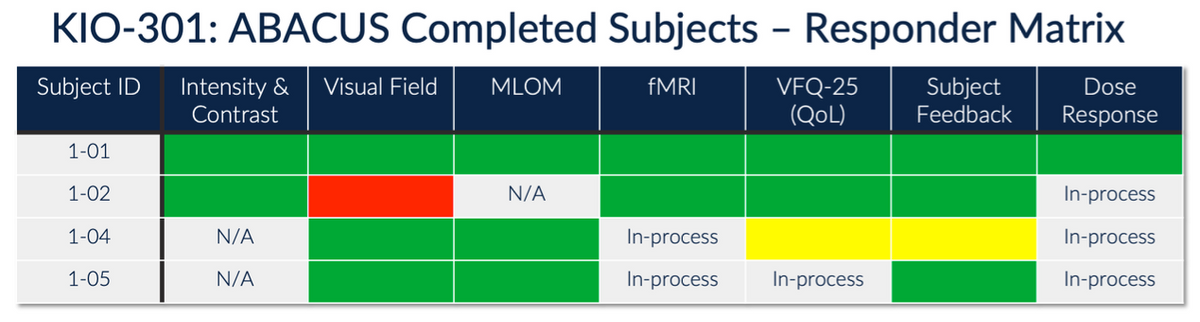
Target enrollment in the Phase 1b study is six subjects (12 eyes) in an open-label, contralateral, dose-escalation format. The initial cohort of a 7.5 μg single IVT injection (low dose) was selected to demonstrate safety and tolerability. Excitingly, initial results presented at the ARVO meeting in April 2023 also suggest signs of efficacy.
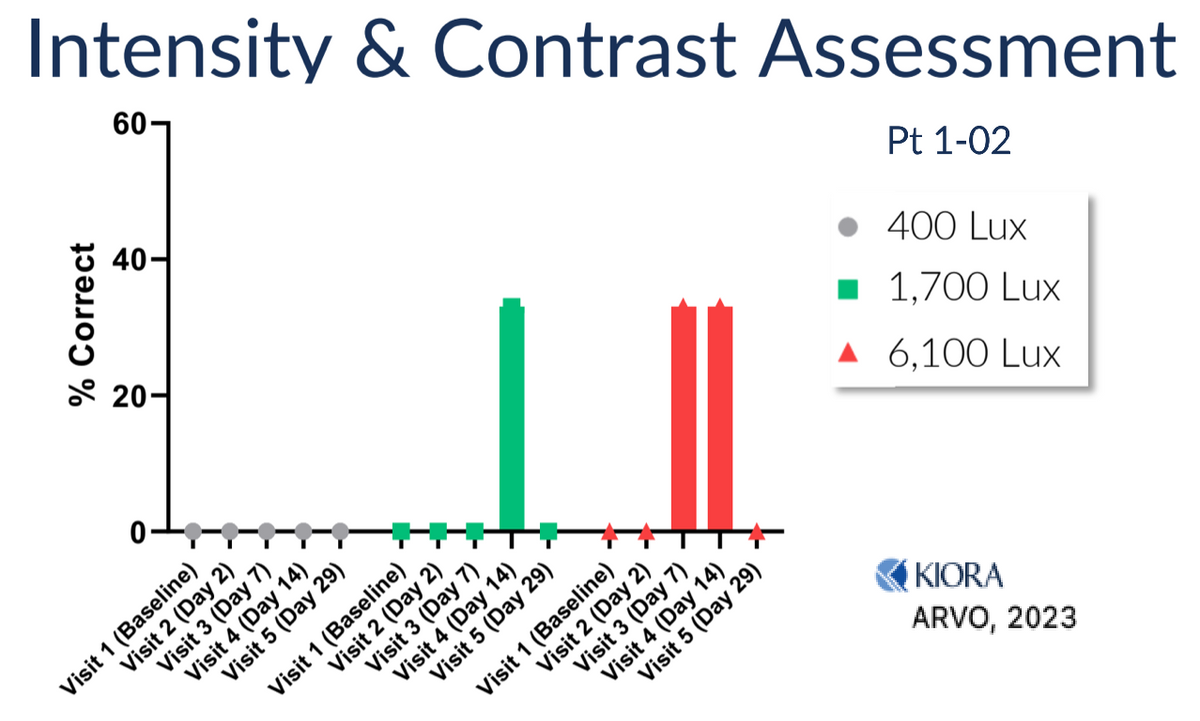
While first-in-human studies are designed to primarily be a safety and pharmacokinetic study, Kiora is measuring efficacy in a number of objective and subjective ways, including the Goldman Perimetry and the Berkeley Rudimentary Vision Test (BRVT). One way the company measures efficacy is a basic light perception test where the subject sits in front of a projector, and increasing light intensities are projected onto the screen. The patient reports if/when they can see the light. Results from an initial patient with no light perception at any intensity at baseline show encouraging results, with the patient able to see light initially at day 7 with very bright light and at day 14 at bright and mid levels of light. The patient’s vision returned to baseline in this assessment - no light perception - by Day-29, which is consistent with the expected pharmacokinetic profile of KIO-301.
Kiora believes that KIO-301 will likely require monthly dosing, although data from the higher dose cohorts have yet to be presented, and the potential exists to develop extended-release formulations or package KIO-301 into sustained-release delivery vehicles to extend the regimen.
Below is an image of one of the Phase 1b subjects undergoing the light perception test. The subject had no ability to see the light at baseline and reports being able to see the flashing light on the screen on Day-7 after the initial dose of KIO-301.

Subjects also showed improvement on the Kinetic Visual Field (Goldman Haag-Streit) assessment, which measures total horizontal and vertical visual perception.
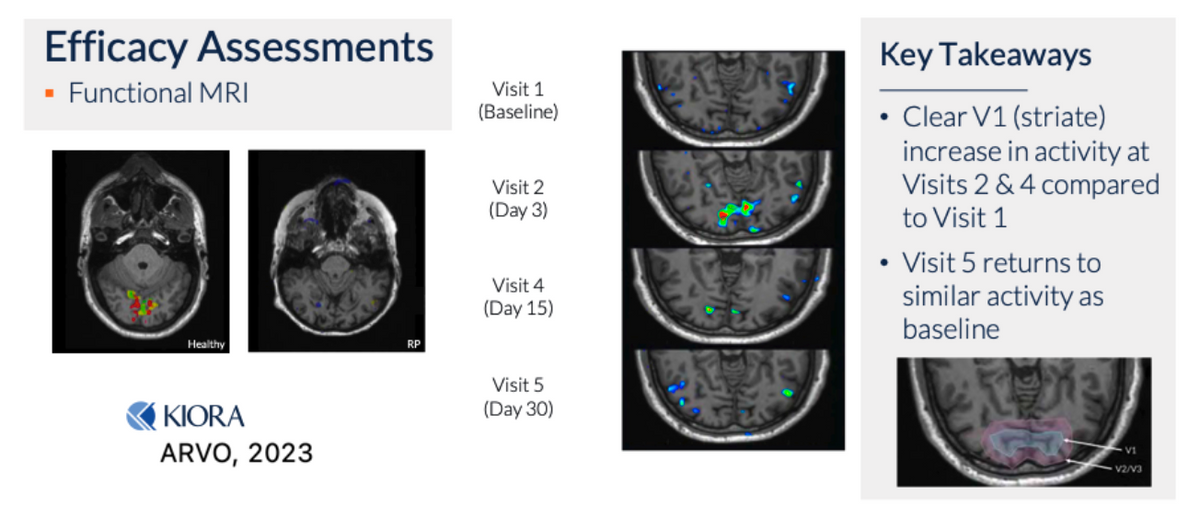
Another way the company is measuring efficacy is by functional MRI, essentially looking at the visual cortex in the brain to see if there is a signal activity in the presence of light - a truly objective measure, as patients cannot fake brain activity. The baseline MRI scans for patients with RP, especially NLP/BLP patients, show no activity in the V1 region of the brain upon light perception tests. However, subsequent to a single IVT of KIO-301, brain activity upon light perception tests becomes evident.
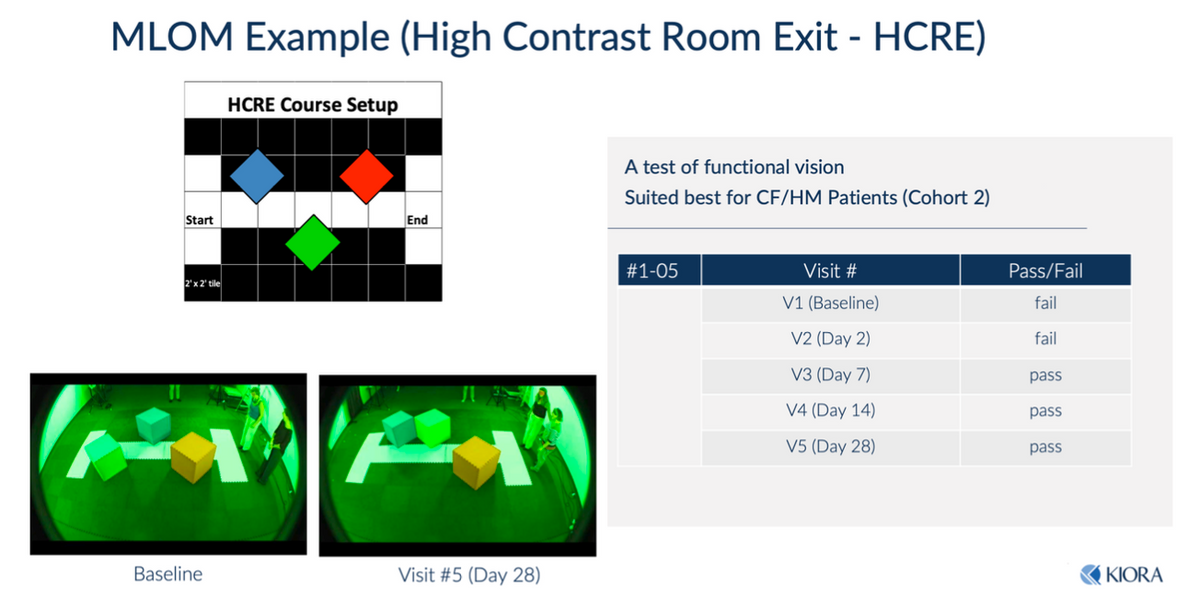
Another way to objectively test the efficacy is the dark room maze test, also known as the High Contrast Room Exit (HCRE test). One subject at baseline and Day-2 failed attempts to navigate the maze. However, subsequent to KIO-301 dosing, the subject was able to navigate the maze successfully on Days-7, -14, and -28 (note the configuration of the maze changes each test).
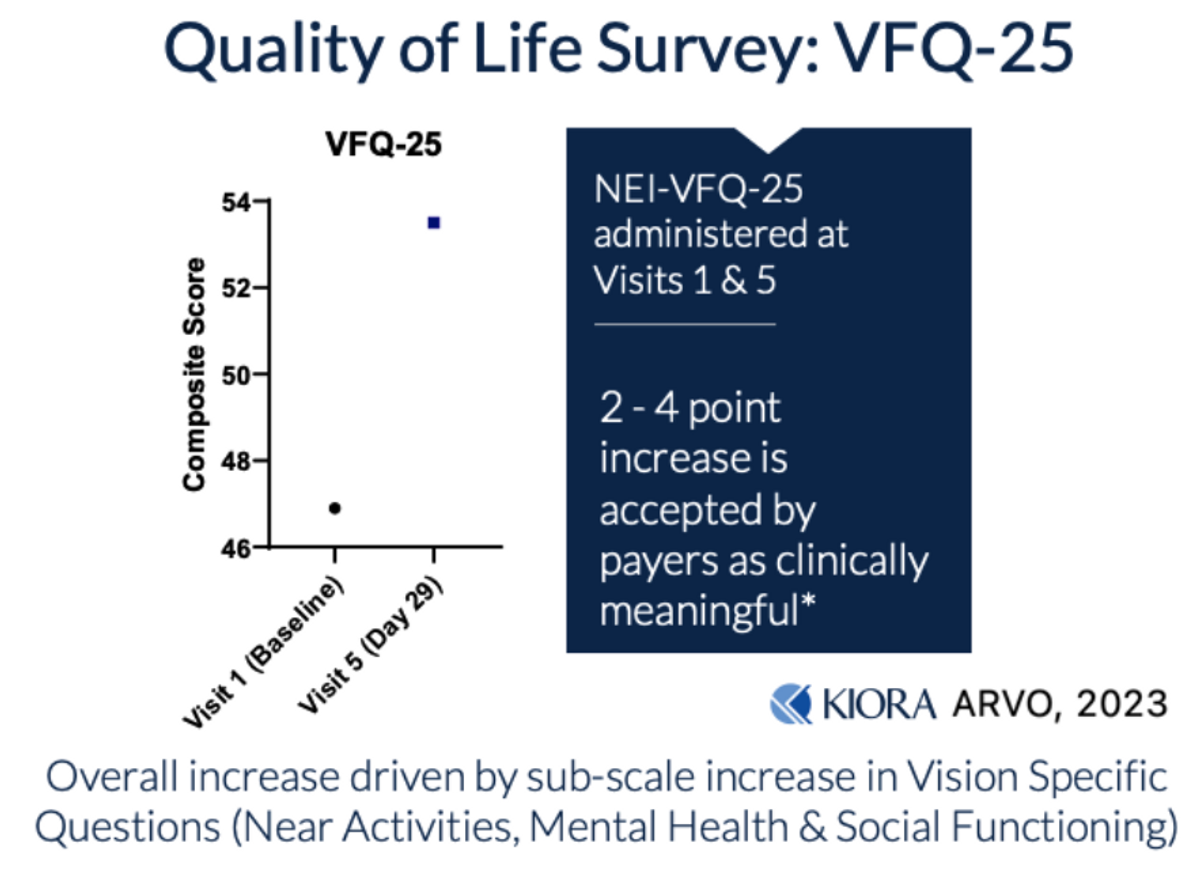
Kiora is also assessing the quality of life (QoL) improvements in subjects participating in the Phase 1b study, clearly a subjective measure, but initial results suggest an improvement.
Here’s a video of one of the subjects talking about improvements in QoL following initial dosing with KIO-301. The subject reports being able to see a flashing light as soon as 48 hours after his initial dose of KIO-301. Another subject reports being able to see light and shapes, including her dog for the first time...
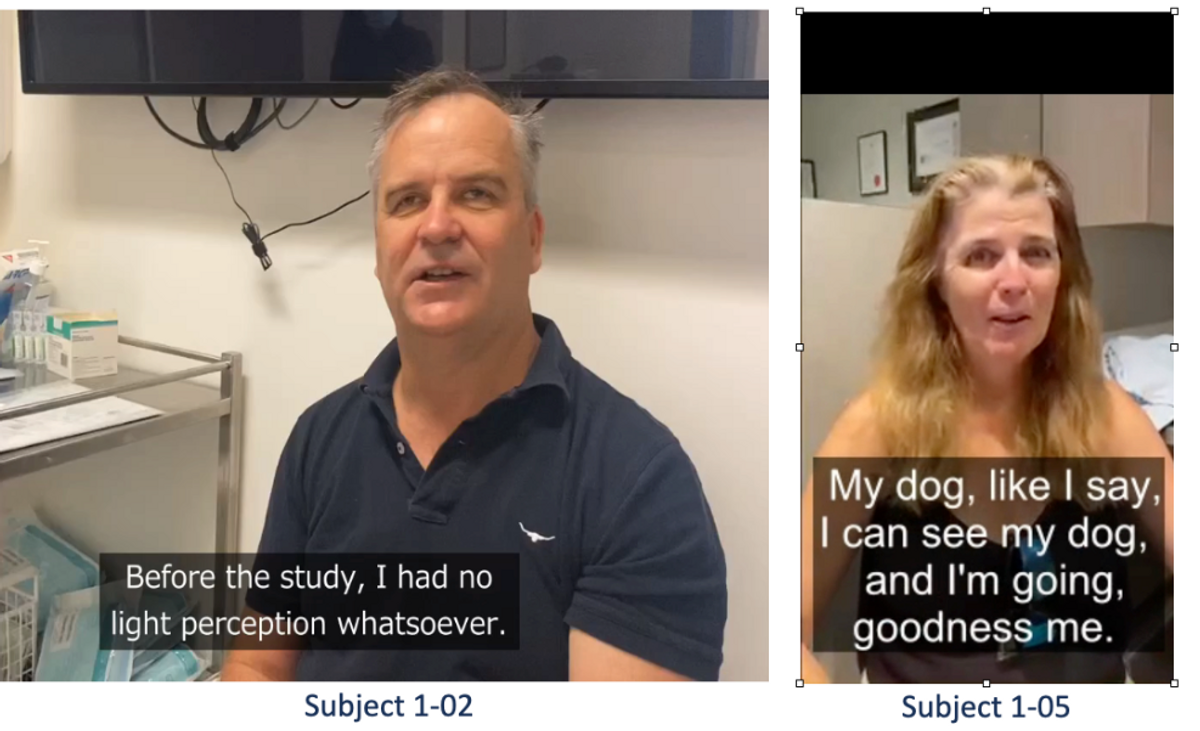

The videos linked above are “illuminating” (pun intended). Initial results to date suggest KIO-301 is safe, well-tolerated, and showing signs of efficacy in a historically difficult disease to show improvement. In fact, all patients treated to date demonstrated some sort of objective and subjective response, with the response appearing to be more robust than initially hoped in the lowest dose cohort, which enrolled the most difficult-to-treat - virtually blind - subjects. Additional data are expected later this year.
RP is a rare, degenerative, inherited retinal disease, with many subjects progressing to the point of total blindness by the later stages of life. A genetic mutation causes the disease, and nearly 100 different mutations have been linked to the disease to date. The only U.S. FDA-approved treatment option, a gene therapy called Luxturna® that costs $450,000 per eyeball, is approved for a specific mutation in the RPE65 gene. This mutation is present in about 0.3-1% of all RP patients. Roche does not break out sales of Luxturna®, but they are reported to be in the area of $300-400 million for the company.
A drug such as KIO-301, which is not specifically targeted to any mutation, not gene therapy, and so not expected to cost an exorbitant amount, is a small molecule with chronic dosing, and so it should provide a safer, more tolerable approach, with potential to discontinue upon any serious adverse event, could be a game-changer for RP patients. It could truly light up the eyes of these patients.
Forward-Looking Statements