In early August 2023, Kiora Pharmaceuticals, Inc. (NASDAQ: KPRX) announced a sharpened clinical development focus on rare retinal diseases with large unmet needs, initially dedicating attention to inherited retinal diseases (IRDs). Kiora recently completed a Phase 1b study of KIO-301 treating patients with late-stage Retinitis Pigmentosa (RP), with targeted indication expansion into two additional orphan IRDs, Choroideremia (CHM) and Stargardt Disease (SD). KIO-301 is a small molecule photoswitch that has the potential to meaningfully improve vision in late-stage IRD patients with photoreceptor dysfunction. KIO-301 represents a beneficial and cost-effective treatment option for several orphan retinal diseases where there is significant unmet medical need.
Initial KIO-301 Data Shines
Preliminary data from the Phase 1b study investigating KIO-301 in patients with RP (ABACUS) was presented at the ARVO 2023 Annual Meeting in April 2023. Initial data showed that a single intravitreal injection of KIO-301 resulted in on-target pharmacology with excellent safety and tolerability. Further, and more exciting, is that all patients in the study demonstrated some form of objective or subjective response to KIO-301. Most patients showed improvement in peripheral vision (Goldmann Perimetry) and successful navigation in the Ora-MLOMTM (Multiluminance Orientation and Mobility) High Contrast Room Exit, a test of mobility and functional vision under controlled lighting. Light perception improved from baseline in patients with no or bare light perception (NLP and BLP respectively).
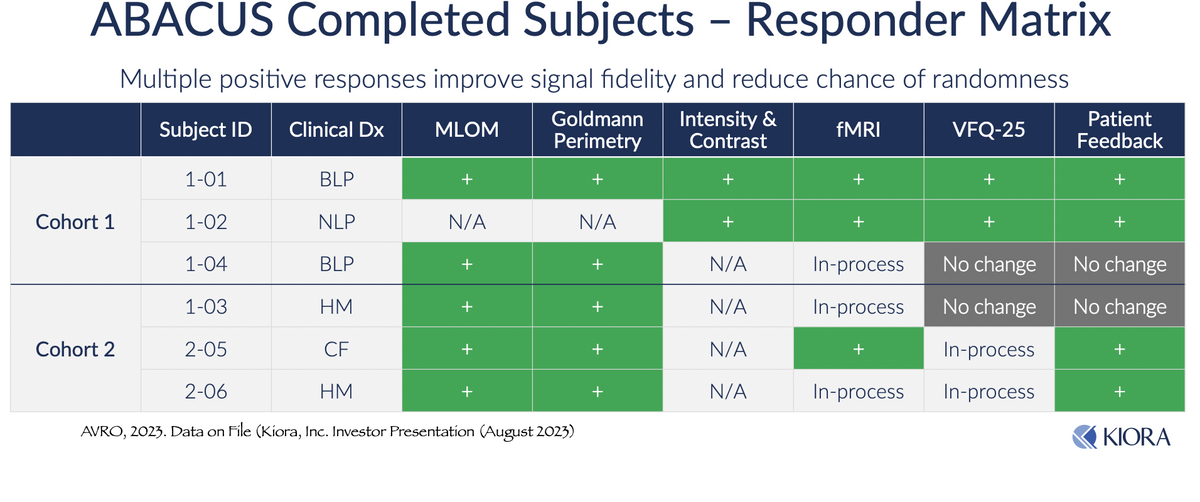
Patient-reported feedback has been positive, with several patients noting improvement in the use of sight in common daily activities and in quality of life, as measured by the National Eye Institute Visual Function Questionnaire (VFQ-25). Kiora will provide additional data from ABACUS during the Retina Subspecialty Day at the American Academy of Ophthalmology annual conference (AAO 2023) in November 2023.

Photoswitch Mechanism - Let There Be Light!
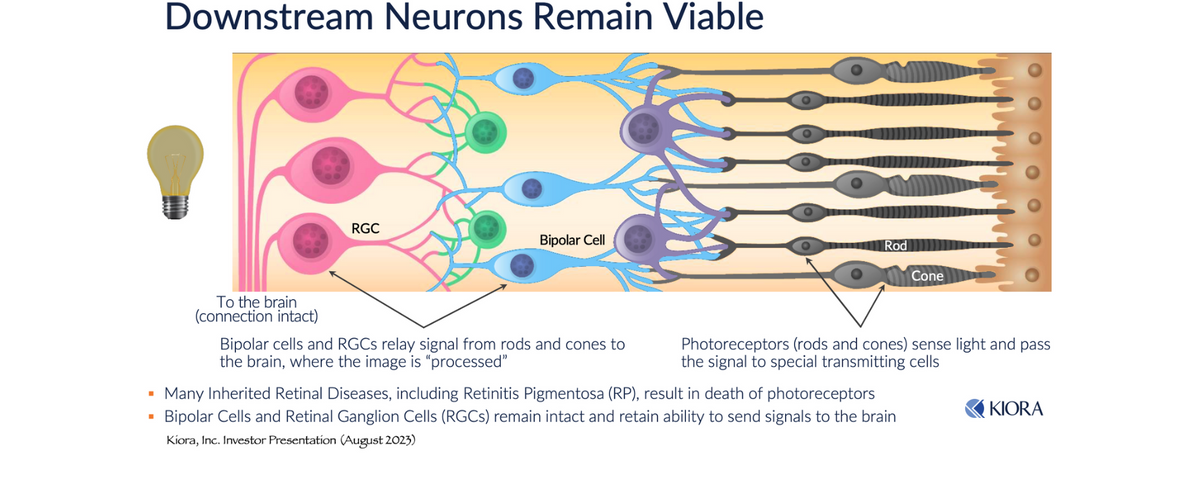
In many progressive IRDs, including RP, CHM, and SD, the loss of vision is brought on by the death of photoreceptors (rods and cones). These are the cells that sense light and convert that signal into the type of electrical signal that is required by the brain to process vision. In healthy individuals, this signal is passed via bipolar cells to retinal ganglion cells (RGCs). RGCs form the optic nerve, which transmits the signal to the vision-processing center of the brain. In patients with many types of IRDs (including RP, CHM, and SD) the death of photoreceptor cells occurs; however, bipolar cells and RGCs remain viable. Ion channels on the cell surface of the RGCs, including K+ channels (voltage-gated potassium channels) and HCN channels (hyperpolarization-activated cyclic nucleotide-gated channels) regulate how these cells communicate with the brain. These channels set the resting membrane potential and control the responsiveness of the cells to synaptic input.
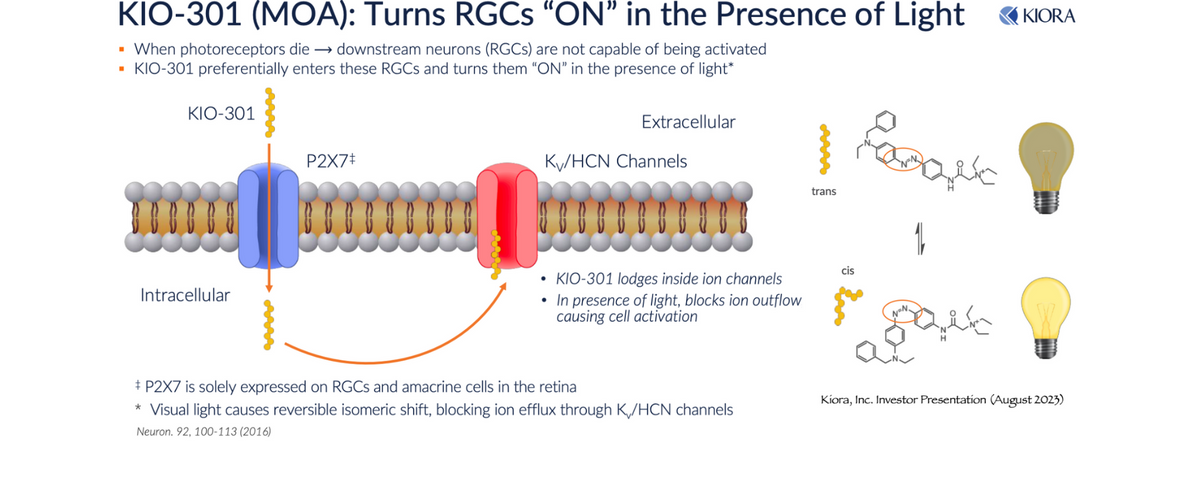
KIO-301, a small molecule referred to as a molecular photoswitch, presents a unique opportunity, turning RGCs into cells capable of detecting light and signaling the brain, bypassing the degenerated rods and cones. The drug preferentially enters RGCs in the retina via the P2X7 transmembrane receptor, which is highly upregulated in the RGCs of diseased retinas. Once inside the cell, KIO-301 lodges inside the Kv/HCN ion channels in a non-covalent (reversible) manner. In the absence of light, KIO-301 is in the low energy (“linear”) trans-configuration, allowing the ion channel's normal flow. This results in the OFF state for RGCs. However, in the presence of light, KIO-301 undergoes a reversible isomeric shift to the high energy (“orthogonal”) cis-configuration, blocking ion efflux through Kv/HCN channels. This triggers RGCs to the ON state, sending a signal to the brain that there is light. This process reverses itself once the source of light is lost.
In RP, the progressive death of photoreceptor cells can be caused by over 150 distinct mutations. This is a large market opportunity, as there are currently no treatment options available. RP affects approximately 1 in 4,000 people around the world, which puts the target market in the U.S. at roughly 100,000 patients. KIO-301 offers several unique characteristics that differentiate the asset from competitors:
- Late-stage disease: KIO-301, based on its mechanism of action, has the potential to be applied to late-stage IRD patients. Future clinical development will initially focus on this population, as the RGCs in these patients express more entry points for KIO-301. Conversely, gene therapies in development seek to stop progression for a small subset of patients.
- Mutation agnostic: KIO-301 has the potential to be used in any one of the more than 150 gene mutations associated with RP and other IRDs as a standalone solution or as an adjunct with future gene therapies.
- Delivery: KIO-301 is administered via intravitreal injection (IVT), which allows for in-office administration (rather than a surgical suite) with significantly less patient discomfort and lower costs than the delivery routes taken by multiple other therapeutics in development.
- Cost: KIO-301 is a small molecule that can be manufactured and provided to patients at a reduced cost (compared to the gene therapy Luxturna®, which costs roughly $450,000 per eye).
- Convenience: The existing formulation of KIO-301 will likely require monthly to bi-monthly dosing; however, Kiora believes that the use of sustained-release delivery vehicles could extend dosing well beyond monthly, which would be a great convenience for patients.
Expanding into Additional Orphan IRDs
There are several other IRDs with pathophysiology resulting from the death of photoreceptors including Choroideremia (CHM), an X-linked recessive disease affecting approximately 1 in 50,000 individuals (~12,000 in the U.S.). It is caused by a mutation in the gene encoding Rab escort protein-1 (REP1). Initial symptoms include loss of night vision leading to severe vision impairment and complete blindness. Similar to RP, despite the death of photoreceptors, bipolar cells and RGCs remain viable. KIO-301 has the potential to return vision to these patients. Onset is typically between the ages of 5 and 16 years of age, and there are no approved treatment options.
Kiora is planning to evaluate KIO-301 in up to 3 patients with CHM as part of a clinical trial expansion over the coming months. Kiora is collaborating with the Choroideremia Research Foundation (CRF), the largest global not-for-profit organization focused on finding a cure for Choroideremia, to create education and awareness for KIO-301, assist in protocol and trial design, and help identify patients for possible participation in the upcoming clinical trials.
Another IRD caused by an inherited gene mutation that results in the death of photoreceptors while sparing bipolar cells and RGCs is Stargardt Disease (SD). A mutation in the ABCA4 or ELOVL4 genes causes the disease, which manifests as lipofusion plaques in the retinal pigment epithelium (layer of the retina), leading to inflammation and photoreceptor cell death. Onset is typically in the 20s, with progressive central vision loss by age 40 or 50. SD affects approximately 1 in 8,000 to 10,000 individuals worldwide (~30,000 in the U.S.), with no approved treatment options available. Kiora is planning a Phase 2 study in SD to start in the first half of 2024.
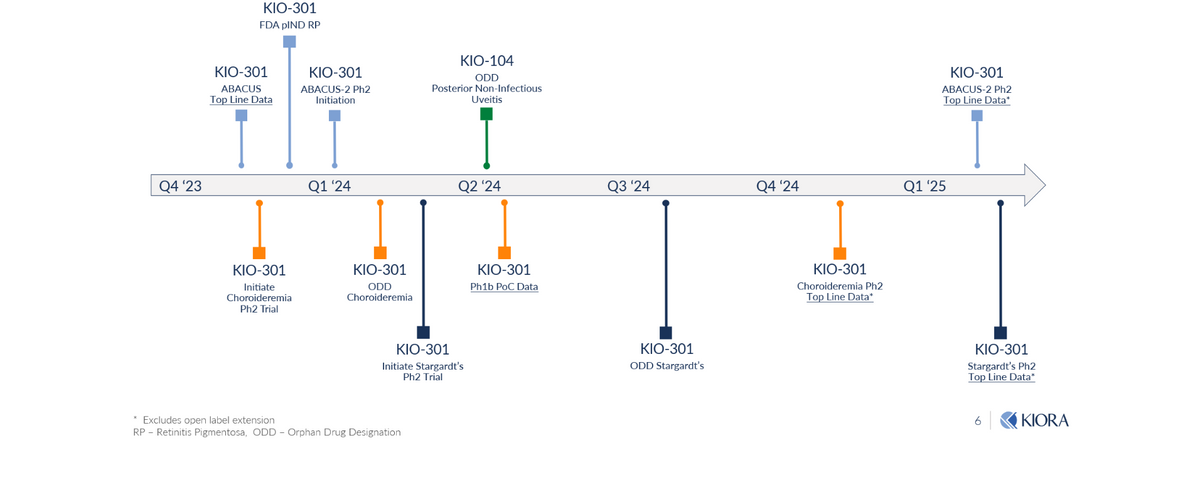
Milestones to Watch
Kiora has a number of upcoming milestones investors should watch for, including a full presentation of the Phase 1b KIO-301 study in RP at AAO 2023 on November 4, 2023, followed by the initiation of several Phase 2 KIO-301 studies over subsequent months in RP, CHM, and SD.
KIO-301 presents an exciting and novel approach to possibly restore meaningful vision back to patients with inherited retinal diseases. It is a first-in-class therapeutic with the potential to restore vision across several inherited retinal diseases, regardless of underlying gene mutations, in an easy to deliver and cost conscious approach.
Kiora Pharmaceuticals is a clinical-stage biotechnology company developing and commercializing products for the treatment of ophthalmic diseases. KIO-301 is being developed for the treatment of retinitis pigmentosa. It is a molecular photoswitch that has the potential to restore vision in patients with inherited and/or age-related retinal degeneration. KIO-101 is being developed for the treatment of the Ocular Presentation of Rheumatoid Arthritis (OPRA). It is a next-generation, non-steroidal, immuno-modulatory and small molecule inhibitor of Dihydroorotate Dehydrogenase (DHODH) with what Kiora believes is best-in-class picomolar potency and a validated immune modulating mechanism (blocks T cell proliferation and proinflammatory cytokine release) designed to overcome the off-target side effects and safety issues associated with commercially available DHODH inhibitors. In addition, Kiora is developing KIO-201, a chemically cross-linked form of the natural polymer hyaluronic acid, designed to accelerate corneal wound healing.
Forward-Looking Statements
Some of the statements in this press release are "forward-looking" and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. These "forward-looking" statements include statements relating to, among other things, the amount of any additional research and development tax incentive credits that Kiora may be eligible to receive, the ability of KIO-301 to restore visual function in patients with retinitis pigmentosa, the potential for KIO-301 to address other eye diseases, the development and commercialization efforts and other regulatory or marketing approval efforts pertaining to Kiora's development-stage products, including KIO-201, as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all. These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this press release, including, among other things, the ability to conduct clinical trials on a timely basis, market and other conditions and certain risk factors described under the heading "Risk Factors" contained in Kiora's Annual Report on Form 10-K filed with the SEC on March 23, 2023 or described in Kiora's other public filings. Kiora's results may also be affected by factors of which Kiora is not currently aware. The forward-looking statements in this press release speak only as of the date of this press release. Kiora expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions, or circumstances on which any such statement is based, except as required by law.